Hot and Cold Chamber Testing for Drug Temperature & Humidity Control
One year after purchasing our product, Rob, a procurement manager at an electronics company, conducted a temperature and humidity calibration and shared his satisfaction: 'Although we haven't been using the chamber frequently lately, I believe it's still performing well.' Let's take a closer look at the Thermal Shock Chamber.
Pharmaceutical stability hinges on precise environmental control during storage and distribution. Hot and cold chamber testing validates drug formulations against temperature fluctuations and varying humidity levels, ensuring active ingredients maintain potency throughout their shelf life. These specialized chambers replicate extreme conditions - from arctic cold to tropical heat - allowing manufacturers to predict degradation patterns, optimize packaging, and meet stringent ICH stability guidelines. By subjecting medications to controlled stress scenarios, pharmaceutical companies safeguard patient safety while demonstrating regulatory compliance across global markets.
Why Temperature and Humidity Control is Critical in Drug Storage?
Chemical Degradation Mechanisms in Pharmaceuticals
Temperature variations trigger hydrolysis, oxidation, and photodegradation reactions that compromise drug efficacy. Aspirin, when exposed to moisture above 60% RH, breaks down into salicylic acid and acetic acid, producing a characteristic vinegar odor. Protein-based biologics denature at elevated temperatures, losing their three-dimensional structure and therapeutic properties. Heat accelerates molecular movement, increasing collision frequency between reactive species and catalyzing unwanted chemical transformations.
moisture above 60% RH, breaks down into salicylic acid and acetic acid, producing a characteristic vinegar odor. Protein-based biologics denature at elevated temperatures, losing their three-dimensional structure and therapeutic properties. Heat accelerates molecular movement, increasing collision frequency between reactive species and catalyzing unwanted chemical transformations.
Moisture-Induced Physical Changes
Humidity directly impacts tablet hardness, dissolution rates, and powder flowability. Hygroscopic excipients absorb atmospheric moisture, causing caking in granules or softening in capsules. Moisture infiltration promotes microbial proliferation in liquid formulations, compromising sterility. Lyophilized products require storage below 25% RH to prevent reconstitution before use, while some sustained-release coatings lose integrity when exposed to fluctuating humidity levels.
Patient Safety and Treatment Efficacy
Subpotent medications deliver insufficient therapeutic doses, prolonging illness or allowing disease progression. Degraded compounds may generate toxic byproducts - penicillin breakdown produces allergenic substances that trigger hypersensitivity reactions. Temperature excursions during shipping to tropical regions have caused insulin analog precipitation, rendering injections ineffective for diabetic patients. Stringent environmental control protects vulnerable populations from treatment failures and adverse reactions.
Key Parameters in Hot and Cold Chamber Drug Testing
Temperature Range Selection and Specifications
Temperature Zone | Application | Typical Range |
Refrigerated Storage | Vaccines, Biologics | 2°C to 8°C |
Controlled Room Temperature | Tablets, Capsules | 15°C to 25°C |
Accelerated Testing | Stability Prediction | 40°C to 50°C |
Extreme Stress Testing | Shipping Validation | -40°C to 60°C |
Modern hot and cold chambers achieve temperature fluctuation within ±0.5°C and deviation below ±2.0°C, matching pharmaceutical stability protocol requirements. The 1°C/min cooling rate and 3°C/min heating rate enable controlled thermal transitions without shocking sensitive formulations. French TECUMSEH compressors deliver reliable performance across -70°C to +150°C spectrum, accommodating both cryogenic preservation studies and heat-resistant packaging evaluations.
Humidity Control and Monitoring Systems
Relative humidity parameters span 20% to 98% RH with ±2.5% deviation tolerance. External isolation humidifiers prevent cross-contamination between samples while stainless steel surface evaporation technology ensures uniform moisture distribution. Automatic water purification systems eliminate mineral deposits that could interfere with humidity sensors. Programmable controllers synchronize temperature and humidity profiles, creating complex environmental cycles that mirror seasonal variations or transportation routes.
Calibration and Validation Requirements
Chambers undergo quarterly calibration using NIST-traceable sensors positioned throughout the working volume. Mapping studies identify hot spots and cold zones, verifying spatial uniformity. Temperature probes placed at geometric centers and corners confirm that all sample locations experience identical conditions. Humidity verification employs saturated salt solutions as reference standards. Documentation packages include calibration certificates, validation protocols, and equipment qualification reports that satisfy FDA 21 CFR Part 11 electronic record requirements.
Simulating Real-world Environmental Conditions for Pharmaceuticals
Geographic Climate Zone Replication
Pharmaceutical products destined for equatorial markets undergo testing at 30°C/75% RH, representing tropical storage conditions per ICH Zone IV guidelines. Mediterranean climates require validation at 25°C/60% RH, while temperate regions use 21°C/45% RH baseline conditions. Chambers programmed with diurnal temperature cycling - mimicking day-night fluctuations - reveal photo-stability issues masked by constant-condition testing. Arctic distribution channels demand freeze-thaw cycling between -20°C and 5°C to assess emulsion separation or crystallization in suspensions.
guidelines. Mediterranean climates require validation at 25°C/60% RH, while temperate regions use 21°C/45% RH baseline conditions. Chambers programmed with diurnal temperature cycling - mimicking day-night fluctuations - reveal photo-stability issues masked by constant-condition testing. Arctic distribution channels demand freeze-thaw cycling between -20°C and 5°C to assess emulsion separation or crystallization in suspensions.
Transportation Stress Simulation
Transport Mode | Temperature Challenge | Duration |
Air Cargo | -40°C to 50°C swings | 24-72 hours |
Ocean Shipping | 15°C to 35°C gradients | 2-6 weeks |
Desert Ground Transport | 60°C peak exposure | 8-12 hours |
Chambers replicate cargo hold conditions where medications experience rapid altitude-induced pressure changes combined with temperature extremes. Shake tables integrated with environmental chambers simultaneously apply vibration stress, revealing synergistic degradation effects. Thermal shock protocols alternate between -20°C and 40°C every 6 hours, identifying packaging failures before products reach pharmacy shelves.
Seasonal Variation Modeling
Annual stability programs incorporate chambers cycling through spring humidity spikes, summer heat peaks, autumn dryness, and winter cold. This approach captures cumulative degradation that single-condition testing overlooks. Pharmaceutical warehouses in continental climates may see 40°C summer temperatures drop to -10°C in winter - conditions that hot and cold chambers reproduce across compressed timescales. Seasonal modeling reveals whether protective packaging maintains internal microclimates when external environments fluctuate dramatically.
Accelerated Stability Testing for Drug Formulations
Arrhenius Equation Application in Shelf-Life Prediction
Elevated temperature testing exploits the Arrhenius relationship where reaction rates double with every 10°C increase. Storing samples at 40°C/75% RH for six months generates degradation data equivalent to two years at 25°C, enabling faster market entry. Pharmaceutical scientists plot degradation kinetics at multiple temperatures, extrapolating shelf-life predictions through regression analysis. This mathematical modeling requires validation through real-time studies but provides preliminary stability estimates within months instead of years.
Forced Degradation Study Protocols
Stress Condition | Target Parameter | Analytical Method |
60°C Heat | Thermal Decomposition | HPLC Assay |
80% RH Humidity | Moisture Uptake | Karl Fischer |
Light Exposure | Photodegradation | UV-Vis Spectroscopy |
Chambers subject formulations to 50°C/75% RH for three months, identifying potential degradation pathways and impurity profiles. Oxidative stress chambers incorporate peroxide challenge or oxygen-enriched atmospheres. These aggressive conditions reveal formulation weaknesses - inadequate antioxidants, permeable packaging, or unstable excipient combinations. Degradation products undergo structural characterization, informing quality specifications and analytical method development.
Statistical Analysis and Confidence Intervals
Stability data requires statistical rigor to support regulatory submissions. Multiple chamber units running parallel studies provide replication necessary for meaningful variance calculations. Regression analysis determines degradation rates with 95% confidence intervals. Outlier detection algorithms identify aberrant data points caused by equipment malfunction or sampling errors. Statistical software packages generate shelf-life estimates with upper and lower bounds, accounting for batch-to-batch variability and analytical uncertainty.
Evaluating Active Ingredient Potency under Stress
HPLC Quantification of Degraded Compounds
High-performance liquid chromatography separates parent drug from degradation products, quantifying potency loss over time. Samples withdrawn at predetermined intervals undergo dissolution testing followed by chromatographic analysis. Method validation confirms linearity across 80% to 120% of labeled claim, with precision below 2% RSD. Stability-indicating methods distinguish between active pharmaceutical ingredient and structurally related impurities, meeting USP <1225> requirements.
Physical Property Assessment Techniques
Beyond chemical assay, hot and cold chambers enable monitoring of dissolution profiles, hardness evolution, and friability changes. Tablets stored at high humidity may soften, affecting disintegration times. Capsules become brittle under dry conditions, cracking during handling. Appearance attributes - color shifts, coating defects, or phase separation - signal formulation instability. Texture analyzers quantify mechanical strength degradation, correlating environmental exposure with compromised quality attributes.
Bioavailability Implications of Degradation
Potency reduction below 90% of labeled claim renders medications subtherapeutic. Crystalline form conversions alter dissolution kinetics, changing absorption rates in the gastrointestinal tract. Enantiomeric purity shifts may produce inactive or toxic stereoisomers. Accelerated testing identifies these critical quality attributes before distribution, preventing therapeutic failures. Bioequivalence studies sometimes repeat with aged samples to confirm pharmacokinetic equivalence throughout product shelf life.
Ensuring Regulatory Compliance in Drug Storage
ICH Stability Guideline Implementation
ICH Q1A(R2) mandates long-term testing at 25°C/60% RH for 12 months minimum, with accelerated studies at 40°C/75% RH for six months. Chambers meeting these specifications generate data acceptable to FDA, EMA, and WHO regulatory bodies. Intermediate conditions at 30°C/65% RH bridge gaps between standard protocols. Chambers equipped with data logging systems create audit trails documenting every temperature excursion, humidity deviation, or power interruption - critical evidence during regulatory inspections.
Good Manufacturing Practice Alignment
Environmental chambers reside within validated facilities maintaining ISO 14644 cleanroom classifications where appropriate. Standard operating procedures govern chamber loading, sample handling, and condition monitoring. Change control processes document any modifications to testing protocols or equipment parameters. Annual preventive maintenance includes gasket replacement, sensor recalibration, and refrigerant system evaluation. Deviation investigations analyze root causes when chamber performance drifts outside acceptance criteria.
Documentation and Traceability Systems
Electronic data capture systems timestamp all measurements, linking chamber performance to specific product batches. Programmable controllers generate reports showing temperature-humidity profiles throughout study duration. Chain-of-custody records track samples from manufacturing through stability chamber to analytical laboratory. Regulatory submissions include chamber qualification documents, calibration certificates, and deviation reports. This comprehensive documentation satisfies 21 CFR Part 211 requirements for pharmaceutical manufacturing facilities.
Ensure Pharmaceutical Stability with LIB Industry's Precise Hot and Cold Chamber
Advanced Technical Specifications
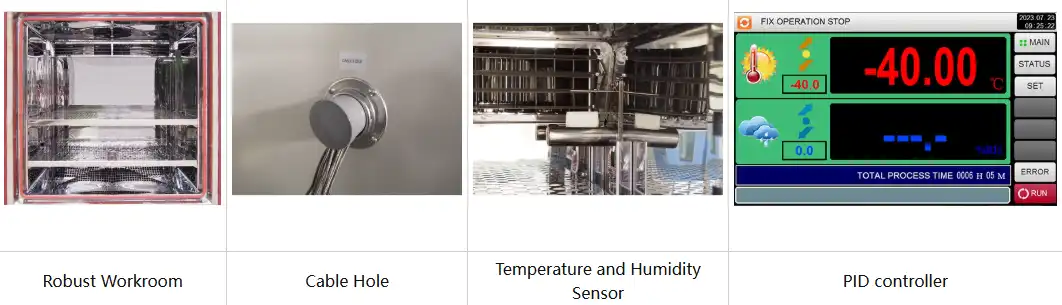
LIB Industry hot and cold chambers feature 304 stainless steel mirror-finish interiors resistant to pharmaceutical compound corrosion. Double- layer tempered glass observation windows with 8cm insulation enable visual monitoring without thermal compromise. Programmable color LCD touchscreen controllers with Ethernet connectivity facilitate remote monitoring and data export. Polyurethane foam insulation maintains thermal efficiency while nichrome heaters provide rapid, uniform temperature distribution. Mechanical compression refrigeration using French TECUMSEH compressors ensures reliable operation across demanding pharmaceutical testing protocols.
layer tempered glass observation windows with 8cm insulation enable visual monitoring without thermal compromise. Programmable color LCD touchscreen controllers with Ethernet connectivity facilitate remote monitoring and data export. Polyurethane foam insulation maintains thermal efficiency while nichrome heaters provide rapid, uniform temperature distribution. Mechanical compression refrigeration using French TECUMSEH compressors ensures reliable operation across demanding pharmaceutical testing protocols.
Comprehensive Safety and Protection Features
Multi-layer safety systems include humidifier dry-combustion protection, over-temperature shutoff, and refrigerant high-pressure alarms. Water shortage sensors prevent humidifier damage while earth leakage protection safeguards personnel. Over-current protection circuits defend against electrical faults. These redundant safety mechanisms minimize downtime and protect valuable pharmaceutical samples from environmental excursions. Automatic water supply systems with purification modules eliminate manual intervention, reducing contamination risks during extended stability studies.
Customization and Service Excellence
LIB Industry provides turnkey solutions encompassing chamber design, installation, commissioning, and operator training. Standard configurations include cable ports with sealing plugs and adjustable shelving, with custom options for specialized sample fixtures. Models ranging from 100L benchtop units to 1000L walk-in chambers accommodate diverse testing volumes. Three-year warranties demonstrate manufacturing confidence while door-to-door delivery service ensures global accessibility. Technical support teams assist with protocol development, troubleshooting, and regulatory compliance questions throughout the product lifecycle.
Conclusion
Hot and cold chamber testing forms the foundation of pharmaceutical quality assurance, validating drug stability across diverse environmental challenges. Precise temperature and humidity control enables accelerated shelf-life predictions, regulatory compliance demonstration, and patient safety protection. Advanced chamber technology replicates real-world stress conditions, revealing formulation vulnerabilities before market distribution. Statistical rigor applied to stability data generates defensible shelf-life claims accepted by global regulatory authorities.
FAQsWhat temperature range is required for ICH-compliant pharmaceutical stability testing?
ICH guidelines specify long-term storage at 25°C±2°C with 60%±5% RH, while accelerated testing requires 40°C±2°C at 75%±5% RH. Chambers must maintain these conditions with minimal fluctuation, typically ±0.5°C temperature stability and ±2.5% humidity deviation to generate regulatory-acceptable data.
How do hot and cold chambers prevent cross-contamination between different drug samples?
Modern chambers utilize external isolation humidification systems and stainless steel construction to minimize contamination risks. Separate shelving, proper spacing between samples, and regular chamber cleaning protocols prevent compound migration. Some facilities dedicate specific chambers to particular drug classes, eliminating cross-contamination concerns entirely.
Can accelerated stability testing completely replace real-time studies for pharmaceutical products?
Accelerated testing predicts shelf life through mathematical modeling but cannot fully replace real-time studies. Regulatory agencies require ongoing real-time data to confirm accelerated predictions and detect long-term degradation pathways that elevated temperatures might not reveal. Both testing approaches complement each other in comprehensive stability programs.
Ready to elevate your pharmaceutical stability testing program? LIB Industry, a leading hot and cold chamber manufacturer and supplier, delivers precision environmental testing solutions tailored to your drug development needs. Contact our technical team at ellen@lib-industry.com to discuss custom chamber configurations and validation support.