How a Shelf Life Test Chamber Supports Drug Shelf Life Studies?
In today’s competitive beverage market, maintaining consistent product quality is essential. Shelf life test chambers offer precise control of temperature and humidity, simulating real-world storage conditions—from hot warehouses to chilled distribution networks. These controlled environments enable manufacturers to evaluate flavor stability, carbonation retention, microbial growth, and packaging performance, ensuring every bottle, can, or carton meets consumer expectations throughout its intended shelf life.
A satisfied client from Russia, specializing in instrumentation, shared: “Our LIB temperature-humidity chamber has been working flawlessly. Everything is excellent, and the chamber performs exactly as expected.” Such feedback underscores the reliability and effectiveness of LIB chambers in supporting beverage quality assurance across a wide range of global applications.
A shelf life test chamber serves as an essential tool for pharmaceutical companies to evaluate medication stability under controlled environmental conditions. These specialized units recreate specific temperature and humidity scenarios that drugs may encounter during storage, transportation, and usage. By exposing pharmaceutical products to accelerated aging conditions, researchers can predict how medications will perform over extended periods, ensuring patient safety and regulatory compliance. This systematic approach allows manufacturers to determine precise expiration dates, optimize packaging materials, and validate storage recommendations before products reach the market.
Why Stability Testing is Critical for Pharmaceuticals?
Pharmaceutical stability testing stands as a non-negotiable requirement in drug development and commercialization. Regulatory agencies worldwide mandate comprehensive stability studies to protect public health and ensure therapeutic efficacy.
comprehensive stability studies to protect public health and ensure therapeutic efficacy.
Regulatory Compliance and Market Authorization
Drug manufacturers must demonstrate that their products maintain quality, safety, and efficacy throughout their intended shelf life. The International Council for Harmonisation (ICH) establishes guidelines requiring stability data before regulatory approval. Without validated shelf life studies, pharmaceutical companies cannot obtain marketing authorization or distribute medications to healthcare providers and patients.
Patient Safety and Therapeutic Effectiveness
Medications that degrade over time may lose potency or develop harmful degradation products. Stability testing identifies potential chemical changes, physical alterations, and microbiological contamination risks. This vigilance prevents patients from receiving ineffective or potentially dangerous medications that no longer meet quality standards.
Economic Implications for Pharmaceutical Industry
Accurate shelf life determination prevents premature product disposal and reduces waste throughout the supply chain. Companies that overestimate stability face product recalls and liability issues, while underestimating shelf life results in unnecessary inventory losses. Precise stability data optimizes manufacturing schedules, inventory management, and distribution strategies.
Temperature and Humidity Control in Drug Testing
Environmental parameters directly influence pharmaceutical stability, making precise control essential for meaningful shelf life studies. Modern test chambers provide the accuracy needed to simulate diverse storage conditions.
provide the accuracy needed to simulate diverse storage conditions.
Precision Environmental Simulation
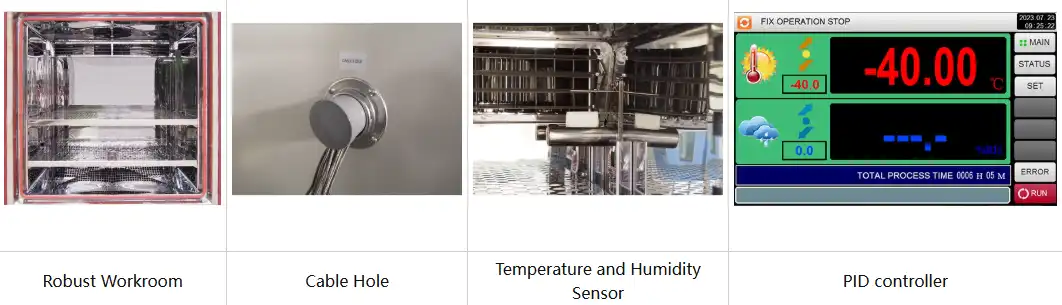
Advanced shelf life test chambers like the TH-225 model offer temperature ranges from -86°C to +150°C with minimal deviation (±2.0°C) and fluctuation (±0.5°C). Humidity control spans 20% to 98% RH, enabling researchers to replicate tropical, temperate, and refrigerated storage scenarios. This precision ensures reproducible results across multiple testing cycles.
Forced Air Convection Systems
Uniform distribution of temperature and humidity throughout the testing space prevents localized hot spots or cold zones. The forced air convection system in quality test chambers circulates conditioned air continuously, ensuring all product samples experience identical environmental stress. This uniformity eliminates variables that could compromise study validity.
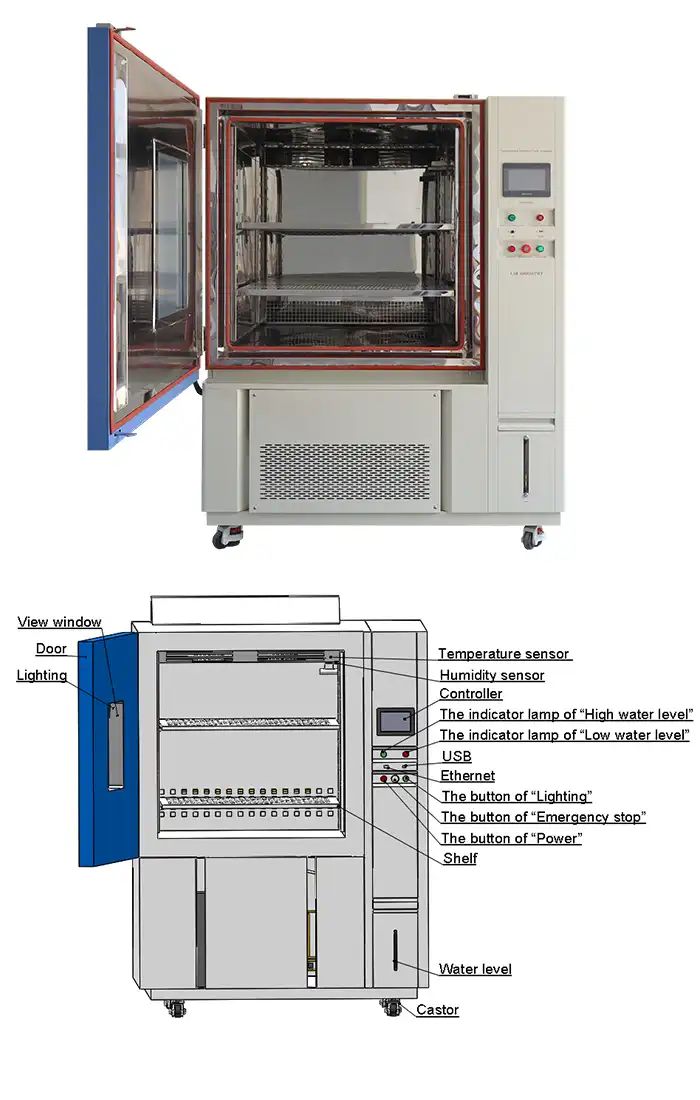
Monitoring and Documentation Capabilities
Programmable controllers with color LCD touchscreen interfaces allow researchers to design complex testing protocols. Ethernet connectivity and USB ports facilitate real-time data monitoring, automated logging, and compliance documentation. These features support Good Manufacturing Practice (GMP) requirements and simplify regulatory submissions.
Environmental Parameter | Testing Requirement | TH-225 Capability |
Temperature Range | -40°C to +150°C | -86°C to +150°C |
Humidity Control | 25% to 75% RH | 20% to 98% RH |
Temperature Uniformity | ±3.0°C | ±2.0°C |
Data Recording | Continuous logging | Ethernet/USB export |
Accelerated and Long-term Drug Shelf Life Studies
Pharmaceutical companies employ different stability testing strategies based on development timelines, regulatory requirements, and product characteristics. Each approach provides distinct insights into medication longevity.
Accelerated Stability Testing Protocols
Accelerated studies expose drugs to elevated temperatures and humidity levels to speed up degradation reactions. Typical conditions include 40°C ± 2°C with 75% ± 5% RH maintained over six months. These stress tests reveal potential stability issues quickly, allowing formulators to identify weaknesses and optimize formulations before committing to lengthy real-time studies.
Real-time Stability Assessment
Long-term testing occurs under recommended storage conditions, typically 25°C ± 2°C with 60% ± 5% RH, extending up to 60 months depending on proposed shelf life. These studies provide definitive data on product behavior under normal storage and confirm predictions from accelerated testing. Real-time data remains the gold standard for regulatory submissions.
Intermediate Stability Studies
Some pharmaceutical products require testing at intermediate conditions (30°C ± 2°C with 65% ± 5% RH) to address specific regional climatic zones. These studies bridge the gap between accelerated stress testing and long-term monitoring, particularly for markets in subtropical regions where storage conditions differ from temperate zones.
Study Type | Temperature | Humidity | Duration | Purpose |
Accelerated | 40°C ± 2°C | 75% ± 5% RH | 6 months | Rapid degradation assessment |
Intermediate | 30°C ± 2°C | 65% ± 5% RH | 12 months | Regional climate simulation |
Long-term | 25°C ± 2°C | 60% ± 5% RH | 12-60 months | Definitive shelf life validation |
Ensuring Active Ingredient Potency over Time
Maintaining therapeutic concentration of active pharmaceutical ingredients (APIs) throughout a product's shelf life represents the primary goal of stability testing. Degradation pathways must be thoroughly understood and controlled.
Chemical Stability Evaluation
APIs undergo various degradation reactions including hydrolysis, oxidation, photolysis, and isomerization. Shelf life test chambers allow researchers to isolate individual stress factors and identify which environmental conditions trigger degradation. Temperature-controlled studies reveal activation energies for degradation reactions, enabling mathematical modeling of stability.
Physical Stability Monitoring
Beyond chemical integrity, physical properties such as dissolution rate, crystal form, particle size distribution, and appearance must remain consistent. Tablets may experience color changes, capsules might become brittle, and suspensions can undergo phase separation. Controlled environmental testing identifies the temperature and humidity thresholds where physical instability occurs.
Assay Methods and Acceptance Criteria
High-performance liquid chromatography (HPLC), mass spectrometry, and other analytical techniques quantify API concentration at predetermined intervals. Regulatory standards typically require products to maintain 90-110% of labeled potency throughout shelf life. Degradation curves generated from chamber studies predict when products will fall below acceptable limits.
Data Interpretation and Shelf Life Prediction
Raw stability data transforms into actionable shelf life determinations through statistical analysis and kinetic modeling. This scientific approach ensures conservative yet economically viable expiration dates.
| Name | shelf life test chamber | |||||
Model | TH-100 | |||||
Temperature range | -20℃ ~+150 ℃ | |||||
Low type | A: -40℃ B:-70℃ C -86℃ | |||||
Humidity Range | 20%-98%RH | |||||
Temperature deviation | ± 2.0 ℃ | |||||
Heating rate | 3 ℃ / min | |||||
Cooling rate | 1 ℃ / min | |||||
Controller | Programmable color LCD touch screen controller, Multi-language interface, Ethernet , USB | |||||
Exterior material | Steel Plate with protective coating | |||||
Interior material | SUS304 stainless steel | |||||
Standard configuration | 1 Cable hole (Φ 50) with plug; 2 shelves | |||||
Timing Function | 0.1~999.9 (S,M,H) settable | |||||
Arrhenius Equation Applications
The Arrhenius relationship describes how reaction rates increase with temperature, allowing extrapolation from accelerated conditions to room temperature storage. By testing at multiple elevated temperatures, researchers calculate activation energy and predict degradation rates at lower temperatures. This mathematical modeling reduces the time required to establish multi-year shelf lives.
Statistical Analysis and Confidence Intervals
Regulatory agencies expect shelf life proposals to include statistical justification with 95% confidence intervals. Linear regression analysis of potency versus time data identifies degradation trends and intercepts representing the point where products no longer meet specifications. Conservative estimates account for batch-to-batch variability and analytical uncertainty.
Out-of-Specification Investigations
When stability samples fail acceptance criteria, investigators use controlled environment chambers to reproduce failures and identify root causes. Systematic testing of variables such as packaging materials, light exposure, and oxygen permeability determines which factors contribute to premature degradation. Corrective actions might include reformulation, improved packaging, or revised storage recommendations.
Analysis Method | Application | Output |
Arrhenius Modeling | Temperature extrapolation | Predictive shelf life estimate |
Linear Regression | Potency trend analysis | Time to specification failure |
ANOVA | Batch comparison | Manufacturing consistency verification |
Power Compliant Pharma Research with LIB Industry's Reliable Shelf Life Test Chamber
Selecting the appropriate shelf life test chamber significantly impacts study reliability, regulatory compliance, and research efficiency. LIB Industry offers advanced solutions specifically designed for pharmaceutical applications.
Technical Capabilities and Customization
The TH-225 and TH-500 models provide pharmaceutical researchers with precise environmental control across extreme temperature ranges. The environmentally friendly refrigerant system delivers cooling rates of 1°C/min and heating rates of 3°C/min, supporting diverse testing protocols. Custom configurations accommodate specialized requirements including explosion-proof designs for volatile materials, low-pressure simulation for altitude studies, and integrated vibration systems for transportation stability assessment.
Quality Assurance and Validation Support
Mirror-finish stainless steel interiors constructed from #304 grade material ensure easy cleaning and prevent contamination between studies. PT100Ω Class A temperature sensors and precision humidity sensors deliver the accuracy required for GMP compliance. Independent water and electrical systems enhance safety during extended stability studies, while network connectivity facilitates data integrity requirements for regulatory audits.
Global Support and Turn-key Solutions
LIB Environmental Simulation Industry provides comprehensive services from initial consultation through installation and operator training. This turn-key approach includes research collaboration, custom design, manufacturing, commissioning, delivery, installation, and ongoing technical support. International customers benefit from CE certification and responsive technical assistance regardless of location.
Conclusion
Shelf life test chambers represent indispensable infrastructure for pharmaceutical stability programs, enabling precise environmental control that validates medication safety and efficacy. Through accelerated testing, long-term monitoring, and sophisticated data analysis, these chambers help manufacturers establish scientifically justified expiration dates while maintaining regulatory compliance. The integration of advanced temperature and humidity control, automated data logging, and customizable features positions modern testing equipment as strategic assets in pharmaceutical development.
FAQs
What temperature range is required for pharmaceutical stability testing?
Pharmaceutical stability testing typically requires chambers capable of maintaining 25°C ± 2°C for long-term studies, 30°C ± 2°C for intermediate testing, and 40°C ± 2°C for accelerated protocols. Some specialized studies may need refrigerated conditions (5°C ± 3°C) or frozen storage (-20°C).
How long do accelerated stability studies take to predict shelf life?
Accelerated stability studies conducted at 40°C with 75% RH typically run six months and can predict shelf lives up to 24-36 months when combined with Arrhenius modeling. Longer accelerated studies provide more reliable extrapolations for products with proposed shelf lives exceeding three years.
Can one test chamber accommodate multiple stability studies simultaneously?
Advanced shelf life test chambers allow simultaneous testing of multiple products if they require identical environmental conditions. Separate chambers or sequential testing becomes necessary when products demand different temperature and humidity protocols to avoid cross-contamination or compromised study integrity.
LIB Industry stands ready as your trusted shelf life test chamber manufacturer and supplier, delivering reliable equipment that meets international pharmaceutical testing standards. Our factory produces CE-approved chambers with customizable features tailored to your specific research requirements. Contact our team at ellen@lib-industry.com to discuss how our environmental simulation solutions can enhance your drug stability programs and accelerate regulatory approvals.