Using a Shelf Life Test Chamber for ICH Stability Compliance
A year after acquiring our product, Rob, a procurement manager at an electronics company, carried out a Shelf Life Test Chamber. He expressed his satisfaction, noting, 'Even though the chamber hasn't been used much recently, I'm confident it's still working effectively.' Now, let's explore the Shelf Life Test Chamber in detail.
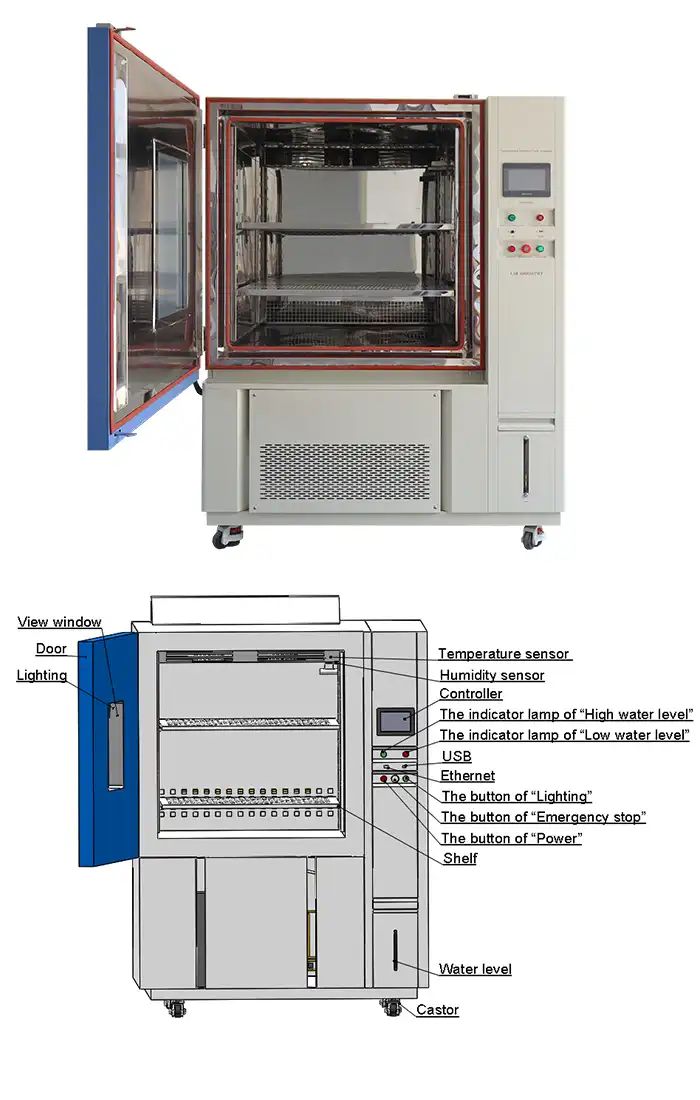
Meeting ICH stability compliance requires precise environmental control to evaluate how pharmaceutical products, medical devices, and nutraceuticals maintain their quality over time. A shelf life test chamber provides the controlled temperature and humidity conditions mandated by ICH guidelines, enabling manufacturers to generate reliable stability data for regulatory submissions. These specialized chambers replicate long-term storage scenarios and accelerated aging conditions, helping companies demonstrate product safety and efficacy throughout the intended shelf life while satisfying stringent requirements from regulatory authorities worldwide.
What are ICH Guidelines for Stability Testing?The Harmonization Framework
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) established unified stability testing protocols to streamline regulatory submissions across different regions. These guidelines eliminate redundant testing by creating standardized methodologies accepted by regulatory bodies in Europe, Japan, the United States, and other participating countries. The framework addresses drug substances, finished pharmaceutical products, and biotechnological materials through comprehensive quality assessment protocols.
Core ICH Stability Protocols
ICH Q1A(R2) defines the fundamental stability testing requirements, specifying storage conditions, testing frequencies, and minimum data requirements. The guideline mandates evaluation under defined temperature and humidity combinations that represent different climatic zones. Zone I encompasses temperate climates, Zone II covers subtropical regions, Zone III represents hot and dry conditions, while Zone IV addresses hot and humid environments. Manufacturers must select appropriate testing conditions based on their intended markets.
Regulatory Acceptance Criteria
Stability studies following ICH guidelines must demonstrate that active pharmaceutical ingredients maintain specified potency levels, physical characteristics remain acceptable, and degradation products stay within established limits. The data generated forms the scientific basis for determining expiration dates, establishing storage recommendations, and supporting shelf life claims on product labels. Regulatory agencies evaluate this information during new drug applications, abbreviated new drug applications, and variation submissions.
Key Parameters in ICH-Compliant TestingTemperature Control Requirements
Precise temperature maintenance represents a critical element of ICH-compliant stability testing. Long-term studies typically require 25°C ± 2°C, while accelerated testing demands 40°C ± 2°C. The shelf life test chamber must maintain these setpoints consistently throughout extended study periods, with minimal deviation. Advanced refrigeration systems using environmentally friendly refrigerants like R404A and R23 ensure reliable performance across the required temperature spectrum from -86°C to +150°C.
while accelerated testing demands 40°C ± 2°C. The shelf life test chamber must maintain these setpoints consistently throughout extended study periods, with minimal deviation. Advanced refrigeration systems using environmentally friendly refrigerants like R404A and R23 ensure reliable performance across the required temperature spectrum from -86°C to +150°C.
Humidity Management Systems
Relative humidity control complements temperature regulation in creating accurate stability conditions. ICH guidelines specify 60% RH ± 5% for long-term testing and 75% RH ± 5% for accelerated studies. Shelf life test chambers employ forced air convection systems with dry and wet bulb humidity sensors to achieve uniform moisture distribution. The 20%-98% RH range available in modern equipment accommodates various testing scenarios, including intermediate conditions and stress testing protocols.
Uniformity and Distribution
Spatial uniformity throughout the testing chamber ensures all samples experience identical environmental exposure. Temperature deviation should not exceed ± 2.0°C between different locations within the workspace. Mirror-finish stainless steel interiors with optimized air circulation patterns minimize hot spots and cold zones. Multiple sample shelves with perforated designs facilitate airflow while accommodating substantial product quantities during simultaneous testing campaigns.
Parameter | Long-Term Condition | Accelerated Condition | Intermediate Condition |
Temperature | 25°C ± 2°C | 40°C ± 2°C | 30°C ± 2°C |
Relative Humidity | 60% ± 5% RH | 75% ± 5% RH | 65% ± 5% RH |
Minimum Duration | 12 months | 6 months | 6 months |
Designing Test Protocols for Regulatory ApprovalSample Selection and Placement
Protocol development begins with representative sample selection from commercial production batches manufactured using validated processes. At minimum, three batches should undergo stability evaluation to demonstrate consistency. Sample placement within the chamber must account for potential microenvironment variations, with positioning documented in the protocol. Rotation schedules may be implemented to eliminate bias from location-specific effects.
Testing Timepoint Determination
ICH guidelines specify minimum testing frequencies for stability programs. Long-term studies require evaluation at 0, 3, 6, 9, 12, 18, and 24 months, with annual testing continuing thereafter. Accelerated studies demand assessment at 0, 1, 2, 3, and 6 months. Intermediate conditions follow a 0, 6, 9, and 12-month schedule. The protocol should define exact timepoint windows and procedures for handling scheduling deviations.
Analytical Method Validation
All analytical procedures used for stability assessment require validation demonstrating specificity, linearity, accuracy, precision, and stability-indicating capability. Methods must detect and quantify degradation products formed under stress conditions. The protocol should reference validated method documents and specify acceptance criteria aligned with product specifications and regulatory commitments. Trending analysis procedures help identify subtle changes preceding specification failures.
Long-term vs Accelerated Stability TestingLong-term Study Characteristics
Long-term stability testing evaluates products under recommended storage conditions over extended periods matching or exceeding the proposed shelf life. This approach generates real-time data reflecting actual stability performance, providing the most reliable foundation for expiration dating. Studies continue at least 12 months beyond the proposed shelf life to demonstrate adequate stability margins. The data supports primary stability claims submitted to regulatory authorities.
| Name | shelf life test chamber | |||||
Model | TH-100 | |||||
Temperature range | -20℃ ~+150 ℃ | |||||
Low type | A: -40℃ B:-70℃ C -86℃ | |||||
Humidity Range | 20%-98%RH | |||||
Temperature deviation | ± 2.0 ℃ | |||||
Heating rate | 3 ℃ / min | |||||
Cooling rate | 1 ℃ / min | |||||
Controller | Programmable color LCD touch screen controller, Multi-language interface, Ethernet , USB | |||||
Exterior material | Steel Plate with protective coating | |||||
Interior material | SUS304 stainless steel | |||||
Standard configuration | 1 Cable hole (Φ 50) with plug; 2 shelves | |||||
Timing Function | 0.1~999.9 (S,M,H) settable | |||||
Accelerated Testing Applications
Accelerated conditions use elevated temperature and humidity to induce faster degradation, predicting long-term stability behavior within compressed timeframes. The Arrhenius relationship between temperature and reaction rate underpins this extrapolation. Significant changes observed during accelerated testing trigger additional intermediate condition studies or necessitate protective packaging modifications. Accelerated data supports preliminary shelf life estimates during product development and provides early warning of potential stability issues.
Combining Study Types
Comprehensive stability programs incorporate both long-term and accelerated testing in complementary roles, typically conducted within a shelf life test chamber to ensure controlled and repeatable environmental conditions. Accelerated studies enable rapid formulation screening and process optimization during development phases. Long-term testing subsequently confirms predicted stability and establishes definitive shelf life claims. The combination provides both timely development insights and robust regulatory data. Ongoing commitment batches enter long-term programs to monitor commercial production stability throughout the product lifecycle.
Study Type | Primary Purpose | Temperature/RH | Duration | Data Application |
Long-term | Real-time stability | 25°C/60% RH | 12-36+ months | Primary shelf life claim |
Accelerated | Predictive assessment | 40°C/75% RH | 6 months | Supporting data, early warning |
Intermediate | Confirmation studies | 30°C/65% RH | 12 months | Additional support when accelerated fails |
Documentation and Reporting for ICH ComplianceProtocol Documentation Requirements
Comprehensive protocols form the foundation of compliant stability programs. Documents must clearly state the study objective, product identification, batch information, storage conditions, sampling schedule, analytical methods, acceptance criteria, and statistical evaluation plans. Deviation procedures, sample tracking systems, and change control processes require detailed description. Regulatory reviewers assess protocol adequacy during application evaluation, making thoroughness essential.
Data Recording and Traceability
Modern programmable controllers with color LCD touchscreens enable automated data logging of chamber conditions throughout study duration. Ethernet connectivity facilitates remote monitoring and data download via USB interfaces. Complete environmental records demonstrate continuous compliance with specified conditions. Any excursions require documentation with impact assessments determining whether affected samples remain suitable for evaluation or require replacement.
Regulatory Submission Formats
Stability reports summarize study results in formats specified by regional regulatory guidance. The Common Technical Document (CTD) format organizes stability data within Module 3 for pharmaceutical applications. Reports should include batch analyses, tabulated results with statistical trending, graphical presentations of key parameters, and scientific interpretation of findings. Conclusions must clearly support the proposed shelf life and storage conditions.
Benefits of Test Chambers in Meeting ICH StandardsPrecision and Reproducibility
Dedicated stability chambers eliminate environmental variability that compromises study integrity. Heating rates of 3°C/min and cooling rates of 1°C/min enable rapid condition transitions between different study phases. Temperature fluctuation within ± 0.5°C ensures setpoint stability during extended operation. This precision generates reproducible data suitable for regulatory decision-making and supports comparability assessments across different study timepoints.
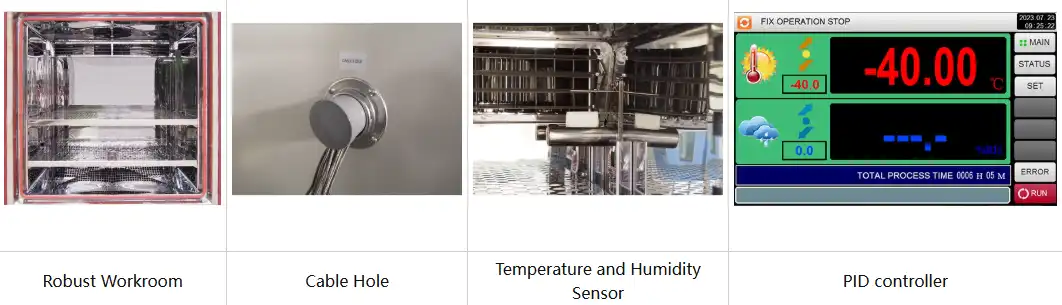
Multi-zone Testing Capability
Advanced shelf life test chambers accommodate simultaneous studies at different ICH conditions, maximizing laboratory efficiency. Independent compartments maintain distinct temperature and humidity combinations, enabling parallel evaluation of long-term, accelerated, and intermediate scenarios. This capability proves particularly valuable during product development when multiple formulations undergo comparative stability assessment. Consolidated testing reduces equipment footprint and operational costs.
Enhanced Safety Features
Equipment design incorporating separated water and electrical systems minimizes safety risks during continuous long-term operation. Mirror-finish stainless steel interiors resist corrosion from humidity exposure and facilitate cleaning when product spillage or contamination occurs. Customization options include explosion-proof configurations for testing volatile formulations and low-pressure chambers simulating high-altitude storage conditions. These features expand testing capabilities while maintaining personnel safety.
Chamber Feature | ICH Compliance Benefit | Operational Advantage |
Programmable controller | Automated condition cycling | Reduced manual intervention |
Network connectivity | Real-time monitoring | Remote oversight capability |
Uniform air circulation | Consistent sample exposure | Eliminates position effects |
Dual refrigeration system | Wide temperature range | Accommodates stress testing |
Simplify ICH Compliance with LIB Industry's Validated Shelf Life Test ChamberProven Performance Specifications
The TH-225 and TH-500 models from Xi'an LIB Environmental Simulation Industry deliver the temperature precision and humidity control demanded by ICH protocols. With internal dimensions accommodating substantial sample quantities (500×600×750mm and 700×800×900mm respectively), these chambers support comprehensive stability programs. The -86°C to +150°C temperature range exceeds ICH requirements, enabling stress testing and specialized applications beyond standard stability conditions.
demanded by ICH protocols. With internal dimensions accommodating substantial sample quantities (500×600×750mm and 700×800×900mm respectively), these chambers support comprehensive stability programs. The -86°C to +150°C temperature range exceeds ICH requirements, enabling stress testing and specialized applications beyond standard stability conditions.
Quality Manufacturing Standards
CE-approved construction demonstrates compliance with international safety and performance standards. High-precision PT100Ω/MV A-class temperature probes provide accurate sensing, while forced air convection ensures uniform distribution throughout the workspace. Environmentally friendly refrigerants align with global sustainability initiatives without compromising performance. Punch-design sample shelves fabricated from #304 mirror-finish stainless steel combine durability with easy maintenance.
Customization and Support
LIB Industry's customization capabilities address unique testing requirements beyond standard configurations. Explosion-proof modifications accommodate flammable materials, low-pressure options simulate transportation or high-altitude conditions, and vibration integration enables combined environmental stress testing. The company provides complete turn-key solutions encompassing research, design, production, commissioning, delivery, installation, and operator training. This comprehensive approach ensures seamless integration of stability testing capabilities into pharmaceutical quality systems.
Conclusion
Achieving ICH stability compliance demands environmental test equipment capable of maintaining precise temperature and humidity conditions throughout extended study periods. Shelf life test chambers provide the controlled environments necessary for generating reliable stability data supporting regulatory submissions worldwide. By combining accurate parameter control, comprehensive documentation capabilities, and validated performance, these specialized chambers enable pharmaceutical manufacturers to demonstrate product quality and establish defensible shelf life claims that satisfy regulatory requirements while protecting patient safety.
FAQsWhat temperature tolerance is acceptable for ICH stability studies?
ICH guidelines permit ± 2°C deviation from the specified storage temperature during long-term and accelerated stability testing. Modern chambers typically achieve tighter control within ± 0.5°C fluctuation, providing additional assurance that samples remain within acceptable environmental ranges throughout the study duration.
How many batches require stability testing for regulatory submissions?
Regulatory authorities generally require stability data from at least three commercial-scale batches manufactured using validated production processes. These batches should represent the proposed commercial formulation and packaging configuration, demonstrating consistency of stability performance across multiple manufacturing campaigns.
Can accelerated stability data replace long-term studies?
Accelerated testing provides supporting evidence but cannot substitute for long-term stability data when establishing primary shelf life claims. Regulatory agencies require real-time stability information at recommended storage conditions for final approval, though accelerated studies help predict behavior and support interim shelf life assignments during product development.
Partner with LIB Industry for Your Stability Testing Needs
As a leading manufacturer and supplier of environmental test chambers, LIB Industry delivers validated shelf life testing solutions that streamline ICH compliance. Our factory produces quality equipment at competitive prices, backed by comprehensive technical support. Contact ellen@lib-industry.com to discuss your stability testing requirements and request a quotation.